
Beyond the darkness, the development of cave habitats are driven by environmental conditions and the availability of energy (nutrients). As there are numerous books devoted to cave ecology (including one of which Jut is editor), this page provides some broad brush strokes concerning how the interplay of these three variables dictate both evolution unfolds and communities are assembled underground.
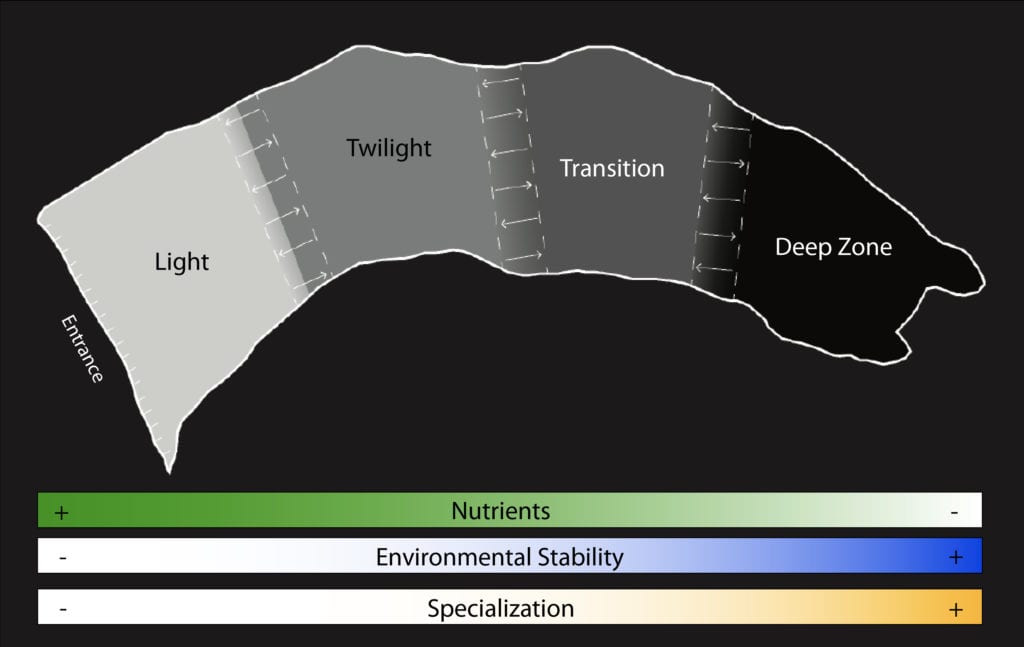
While most caves would be fairly barren environments without energy from the surface, nutrients are deposited randomly along the length of the cave — within the four environment zones. Typically, a cave may be divided into four principal zones. The entrance zone is characterized as a combination of surface and cave environmental conditions. The cave’s twilight zone has both diminished light conditions and climatic influence of surface environment, while the next zone, the transition zone, is an aphotic region where barometric and diurnal shifts are observed at a significantly diminished rate approaching near stable climatic conditions. Finally, the deep zone is completely dark, and largely environmentally stable with a near constant temperature, near water-saturated atmosphere, and low to no airflow.

Photosynthesis fuels most of life on our planet. However, as one traverses beyond the cave’s twilight zone, photosynthesis ceases. Nutrients that reach the deep cave interior are transported from the surface. This has resounding implications on how ecological communities are formed and evolve.
Nutrient types within caves are quite varied. They include bat guano piles, plant roots penetrating the cave from the surface, and flood-transported surface vegetation at one end of the food spectrum with more nebulous sources such as dissolved organic material percolating into the cave with surface water at the other. Regardless of the nutrient type, these energy sources are typically patchily distributed across the four environmental zones of a given cave. The combination of zone and nutrient source can result in the establishment of relatively simple, yet fascinating ecological communities.
As with surface ecosystems, cave habitats undergo a continuum of colonizations and extinction events over time. Organisms with certain pre-adaptive traits are those who successfully colonize the underground realm. Over evolutionary time, an established population may become increasingly better equipped to the cave environment and ultimately the deep zone due to genetic drift, mutations, and selective pressures. This process can result in the development of a troglomorphic (or subterranean-adapted) species.
However, not all species that live in caves are troglomorphic. Cave-dwelling organisms have varying degrees of dependency on the subterranean environment. The most reliant taxa are troglobionts — obligate cave dwellers that can only complete their life cycle within underground, and exhibit morphological and physiological traits indicative of subterranean adaptation (i.e., troglomorphies) and stygobionts, the aquatic counterpart to troglobionts. Obligate troglophiles are taxa seemingly restricted to the subterranean environment, but lack clear traits indicative of troglomorphy. These animals can be tricky to identify. In some cases, they may represent climate relicts, while in others they could be confused with edaphic (soil dwelling) taxa. Troglophiles are species that occur facultatively within caves, can complete their life cycles there, but also occur in similar surface microhabitats. Trogloxenes are organisms that frequently use caves for shelter, but forage on the surface. Organisms that stray into caves, but are evolutionarily ill-equipped to establish viable populations are considered accidental species.
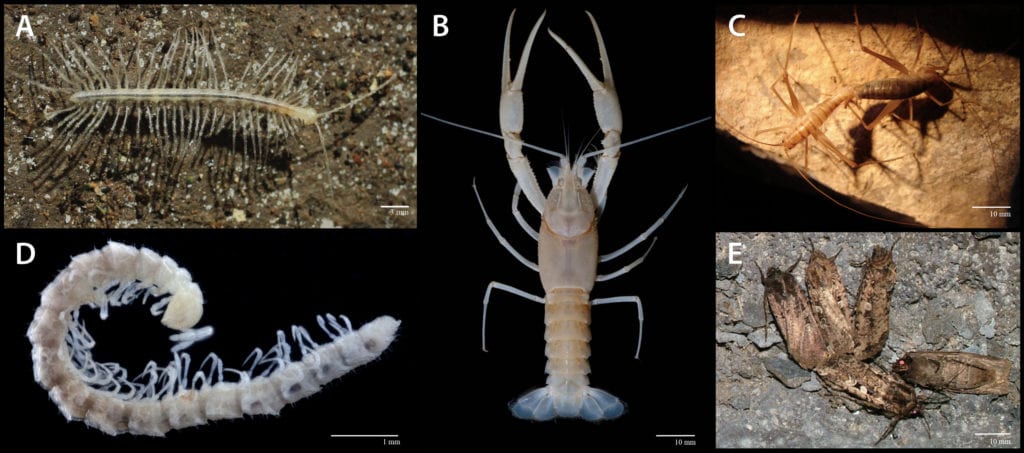
Over the past 50 years, the discipline of cave ecology has flourished. In particular, the past 20 years has witnessed a proliferation of knowledge and understanding of these oft difficult-to-study environments. This leap is due to the advent of geographic information systems for examining biogeographical patterns; a myriad of accessible statistical programs (many of which are freeware) granting robust inference into ecological patterns; and, the warp speed advancement of molecular and genomic techniques that have broadened our understanding related to the evolutionary mechanisms of troglomorphic taxa. Armed with these now contemporary techniques and approaches, our apperception of how the surface environment and nutrients sculpt cave ecosystems will continue to grow with many exciting discoveries evinced along the way.
Going deeper on cave ecosystems and biodiversity…
Further Reading

Wynne, J.J. 2014. Reign of the Red Queen: The future of bats hangs in the balance. The Explorers Journal 92: 40–45.
